Case Study: Defective Screws During ACDF Surgery
How our Litigation Team Investigated our Client’s Failed Surgery, Found the Cause, and Secured a Big Win Without Filing Suit
Summary
Client D.H. was 48 yrs. old and experiencing chronic neck pain, as well as radiating pain and numbness in his arms due to herniated discs. When physical therapy failed to improve his symptoms, he was referred for surgery – specifically an “anterior cervical discectomy and fusion” (ACDF surgery).
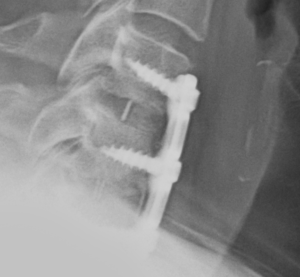
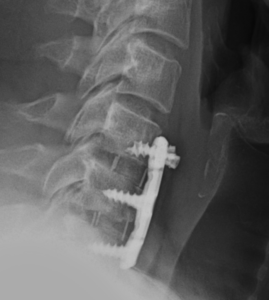
The ACDF surgery involved the surgeon reaching the damaged discs from the front (anterior) of the spine by going through the throat area, moving aside the neck muscles, trachea, and esophagus so that the cervical discs and vertebrae were exposed. The surgeon then removed the damaged discs (discectomy), which left space between the vertebrae empty. Artificial discs were inserted and fixed in place with metal plates and screws.
Client D.H.’s recovery time was about 3 months. At his three-month post-surgical follow-up, imaging of his neck revealed that two of the surgical screws had “backed-out”. It was determined that he had experienced a hardware failure due to a defective medical device and had instability in his neck. He was advised he would need to undergo an immediate revision surgery to remove and replace the faulty surgical hardware.
Client D.H., who had been under a financial strain from the initial ACDF surgery, had just finally fully returned to work and was now being told he would be required to undergo the surgery again as a result of these defective cervical screws. Which means he would have to endure recovery again and, therefore, be off work again. Furthermore, he would be billed for the surgery again.
Client D.H. needed answers as to how this happened, but wasn’t getting any. Approximately 6 months after his second ACDF surgery, he turned to us for help. We not only answered his questions as to how this happened, but we got the defective medical device manufacturer to pay for his surgery, his lost wages, and the pain and suffering he endured, all without having to even file a lawsuit.
The Challenge: Determining if it was a Defective Medical Device
Medical malpractice and medical device litigation are very complex. There is not only a need to understand the complex legal and scientific aspects involved, but also understand the pain and serious side effects clients suffer through in these cases and know how to create an accurate damages model.
Client D.H. experienced unexpected, painful, and serious injuries as a result of a complex surgery that failed. The surgical failure also caused Client D.H. significant financial stress from his lost wages and medical expenses.
“We know how to hold at-fault parties accountable in these cases and bring our clients the resolution they are entitled to.”
Our challenge was to uncover the cause of the failure, identify at-fault parties, and obtain maximum compensation for him as quickly as we could.
How We Determined the Product Was Defective
Our first step was for us to launch an investigation to determine the cause of surgical failure and the resulting defective product. This involved obtaining an extensive medical history from our client and then obtaining all relevant medical records.
“The first step is always to listen to the person who matters most: the Client”
Our team included our medical malpractice attorney, medical device attorney, forensic nurse paralegal, and an orthopedic medical expert.
After we obtained Client D.H.’s medical records and radiology for both ACDF surgeries, we focused on whether the failure was attributable to the client, the surgeon, or the faulty medical device used.
Some surgeries fail, not due to a surgeon error, or medical device failure, but because of a patient’s failure to comply with post-surgical instruction, rehabilitation, or due to other medical conditions the patient may have.
We were able to determine that the records supported that Client D.H. was compliant with all rehabilitation and post-surgical restrictions. Our review of our client’s medical records also did not reveal any medical condition which would affect fusion. Additionally, his second surgery resulted in a successful fusion, supporting the conclusion the failure was not the result of any medical condition.
Client D.H’s operative records indicated that the surgeon ensured that each screw was securely screwed and locked into place. Post-surgical radiology supported this. There was nothing in the medical records themselves that supported surgeon error.
We were able to identity the specific cervical plate and screws used in the initial surgery. With positive product identification we researched the FDA’s medical device database for any recalls, safety communications, or adverse reports concerning the product.
We discovered that numerous complaints had been filed, reporting that the locking mechanism of the surgical screws failed, causing the screws to backout.
Our legal and medical team concluded that the evidence supported product failure.
We shared our findings of the defective locking mechanism and cervical screws with our client and discussed how to proceed. He was very pleased our team discovered what went wrong but had concerns his financial circumstances would worsen throughout the course of lengthy and costly litigation – which in most instances, last years. The financial strain this situation caused him required a more expedited approach.
Armed with medical evidence, radiology images, expert reports, including statements from our Client’s surgeon, and the knowledge that this product had many other reported failures, we decided to concentrate all our efforts on resolving the dispute without filing suit.
We researched jury verdicts and settlement data involving similar injury claims. We again worked with the client to make sure we fully accounted for all his associated losses, including his medical expenses, lost wages, and pain and suffering. Using this information we created a damages model to base settlement on.
We prepared a personalized settlement brochure that was supported with overwhelming evidence that the manufacturer’s product failed and caused his injuries. The settlement demand was effective. It got the medical manufacture’s serious attention.
Result
Thanks to a well-prepared strategy, we were able we clearly demonstrate the faulty medical device manufacturer’s liability, exposure, and our client’s damages, as well as our capability and willingness of taking the company to court if need be.
The medical manufacture’s legal counsel reached out and expressed an interest in sitting down and discussing the claim and seeing whether the parties could come to a resolution.
A team from the company flew out from California to meet in Detroit with us and our client. It was clear the company appreciated significant risk if we moved the claim into court.
After nearly a full day of negotiation, and within less than 6 months from when Client D.H. first reached out to us, we achieved a big win for our client.
“We listened to our Client. We heard his frustrations, pains, and goals. We tailored our strategy and we got the right people in the right place at the right time. Our Client was thrilled with the outcome.”
DISCLAIMER: The results are specific to the facts and legal circumstances of each of the clients’ cases and should not be used to form an expectation that the same results could be obtained for other clients in similar matters without reference to the specific factual and legal circumstances of each client’s case.

